Because the electron structure is the same isotopes have the same chemical properties. 1 See answer Advertisement Advertisement yummcookies is waiting for your help.

Difference Between Isotopes And Elements Compare The Difference Between Similar Terms
Explain how two isotopes of an element are similar.

. Atoms of most of the elements can have several different variations called isotopes. All isotopes have the same number of protons and the same number of electrons. All the Isotopes of an Element Have Identical Chemical Properties.
These different atoms of the same element are called isotopes. Different isotopes have different numbers of neutrons in their nuclei resulting in different atomic weights for the different isotopes of a single element. Atoms of the same element with different numbers of neutrons in the nucleus are referred to as isotopes.
The isotopes of an element are like different versions of an element - they have the same number of protons but different number of neutrons. They are different from each other by having a different number of neutrons. Explain why the isotopes have similar chemical properties but they differ in physical properties.
Thats what an isotope is. 12-carbon will have 6 protons and 6 neutrons while 14-carbon will have 6 protons and 8 neutrons. Chemical properties of different isotopes are almost similar.
Lets use flourine again. This is because the number of electrons determines chemical properties and all three isotopes have one electron in their atoms. Atoms of the same element can be different from each other.
Same element different amounts of neutrons. Isotopes of an element have similar chemical. Some isotopes have medical uses and others are important to nuclear chemistry.
Atoms of the same element can be different. Why Isotopes Have Same Chemical Properties But Different. Isotopes are atomic structures of the same elements having a different mass numberatomic mass.
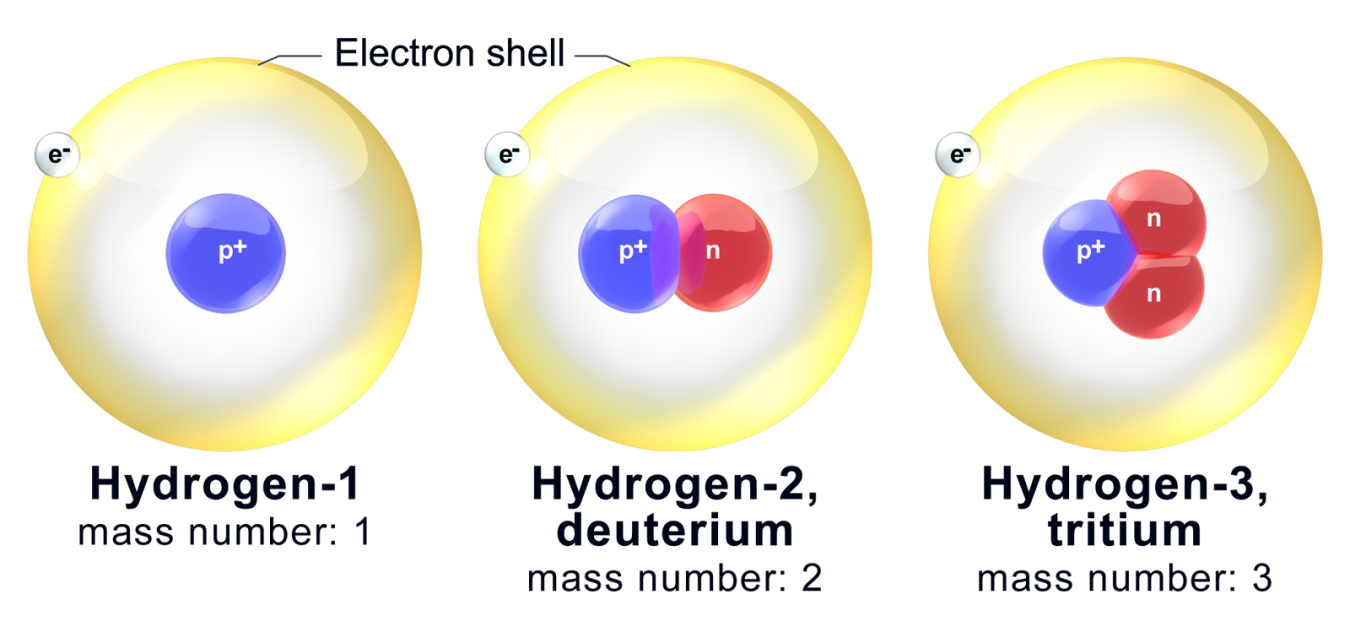
All three isotopes of hydrogen have identical chemical properties. Atomic numbers of isobars vary from each other. Isotopes of an element have different physical properties because they have different mass numbers.
Different isotopes of the same element are chemically the. Explain how all of the isotopes of an element are similar and how they are different. When it comes to physical properties of isotopes including mass melting or boiling point density and freezing point they are all different.
All atoms of a given element have the same number of protons but some atoms have more neutrons than other atoms of the same element and therefore have a. So it is clear that they will have same number of electrons same electronic configuration and thereby same number of valence electrons. Explain how they are different.
Isotopes of an element are those which have similar atomic number but different mass number. What is different is the number of neutrons The different number of neutrons all cause a difference in the atomic weight or mass of the atoms. The isotopes of an element are alike in that they have the same number of protons electrons and the same chemical properties.
All atoms of that element will have the same number of protons but neutrons can vary. The different number of neutrons affects the mass number. 1 Isotopes and Ions Why is this important.
While chemically similar isotopes of the same element will have different masses and may differ in their radioactive properties. Isotopes are defined as one of two or more atoms of the chemical element with the same atomic number but different atomic mass. Since the neutron number is different their mass number also differs.
For example the isotopes of carbon are carbon-12 carbon-13 and carbon-14. These different atoms of the same element are isotopes. The isotopes are different in that they have.
Atomic numbers of all isotopic forms of a single element are equal. Isotopes are the atoms of an element which have same atomic number but different mass number. Yummcookies yummcookies 10122021 Biology High School answered Please answer asap.
The atomic number is defined by the number of protons present in the atom. They are different from each other by having different number of neutrons. Example- 612C and 613C atomic number View the full answer.
An isotope is a chemical structure that is highly similar to its parent isotope with the difference lying in the number of neutrons that the compound has. The differences between isotopes arise. However the isotopes of the same element have the same number of protons.
Isobars are different chemical elements having the same atomic mass. Two different isotopes of the same element have the same number of protons but each has a different number of neutrons in its nucleus. Isotopes have a different number of neutrons but they have the same number of protons and electrons.
Isotope of an element consists of the same number of electrons as any of its electrons within the element itselfHowever they have different numbers of neutrons which in turn result in a different degree of massMass numbers make a difference in physical propertiesAs a consequence many. However the isotopes of the same element have the same number of protons and neutrons. All the Isotopes of an element have identical chemical properties because they have the same number of electrons as an atom of that element but they have different numbers of neutrons.
Isotopes are identified by their mass numbers. As an example carbon has the elemental number 6 which means it has 6 protons. Isotopes of the same element have the same number of protons and electrons when in neutral atomic form.
The physical properties of any isotope are largely determined by its mass. Since the neutron number is different their mass number also differs. Isotopes of the same element are similar by having same number of proton atomic NoThey differ from each other because they posses different mass No.
In turn we can also say that isotopes of an element. They are the same chemical element but their forms are different. The isotopes of an element have the same atomic number but different atomic numbers.
Explain how all of the isotopes of an element are similar and how they are different.

Isotope Definition Types And Examples Chemistry Basics Chemistry Classroom Chemistry Lessons

0 Comments